陈柱成实验室
Chen Zhucheng Laboratory
We use advanced tools in structural biology, such as crystallography and cryo-electron microscopy, in combination with biochemistry, genetics and other methods, to analyze chromatin-related molecular machines.
Structure of human PBAF chromatin remodeling complex bound to the nucleosome
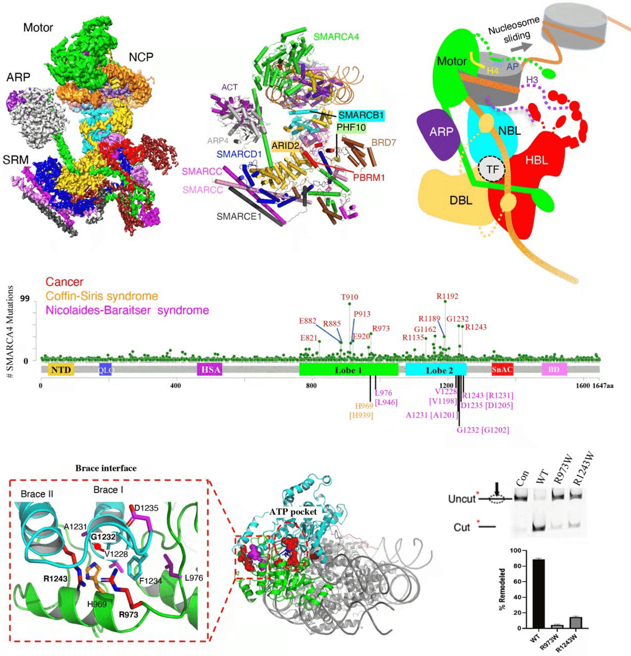
Although the motor proteins remodel nucleosomes in vitro on their own, they function as complexes with multiple auxiliary subunits in vivo to provide regulation and targeting. Snf2 interacts with 11 auxiliary subunits to form the SWI/SNF complex, which regulates the expression of ~5% of the genes in yeast. RSC is a closely related homolog of SWI/SNF, and controls the promoter chromatin architecture of ~70% of the genes in yeast. The yeast SWI/SNF and RSC complexes are highly conserved across evolution, and related to the mammalian SWI/SNF (mSWI/SNF), the BAF and PBAF complexes, respectively.
BAF and PBAF interact with distinct transcription factors, and induce different transcriptional responses. Recent studies of cancer genome sequencing reveal that the genes encoding the BAF and PBAF subunits are mutated in ~20% of the human cancers, and in many intellectual disability syndromes. There is increasing interest in the therapeutic implications of the mutations of the BAF and PBAF subunits.
To illustrate the mechanism of the chromatin remodeling complexes, we determined the structure of RSC bound to the nucleosome in 2019. Our findings shed light on RSC assembly and functionality, and provide a framework to understand the mammalian homologs BAF/PBAF. In 2021, we reconstituted the yeast SWI/SNF complex in vitro, and determined the high-resolution structure bound to the nucleosome, which revealed the detailed structural features of SWI/SNF, and the mechanism of nucleosome recognition. In 2022, we determined the high-resolution structure of the 12-subunit human PBAF bound to the nucleosome. Our findings reveal the structural features of PBAF that distinguish it from BAF, and provide mechanistic insights into nucleosome recognition by the motor subunit. This work also provides a foundation to inform the PBAF-related human diseases, and a structural basis for the drug development in targeting the mSWI/SNF complexes.

扫一扫关注微信